 Extended Stability for Parenteral Drugs
Extended Stability for Parenteral Drugs

The only guide to safely extend the dating of parenteral drugs
Extended Stability for Parenteral Drugs supports safe extended dating of parenteral drugs beyond the usual 24-hour limit – minimising waste and lowering medicines costs. The go-to reference for anyone preparing, administering, or compounding.
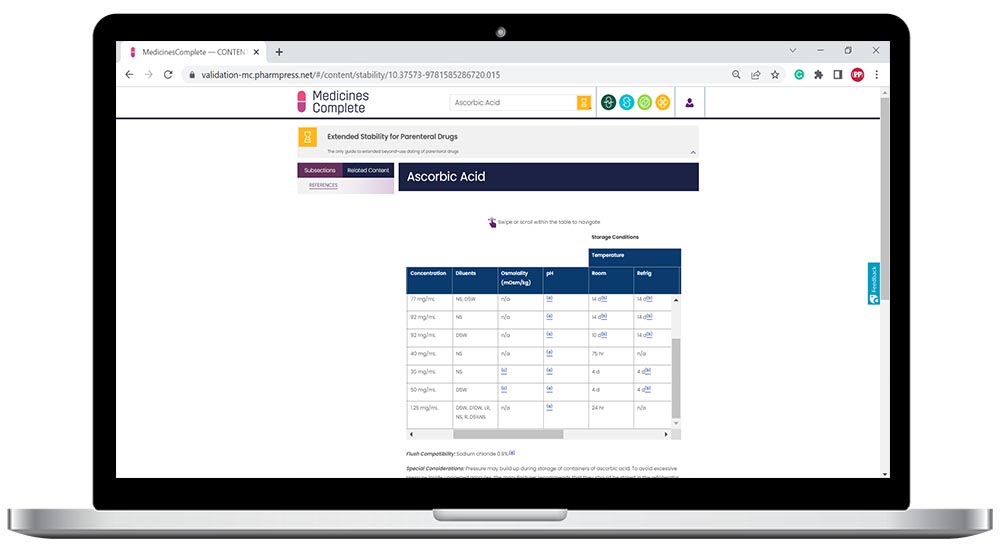
Extended Stability for Parenteral Drugs provides an extensive list of medications with temperature excursion data for intact vials. Coverage of all aspects of determining stability, including the changing elastomeric landscape and the ongoing variability in stability data. Plus, newly published data on parenteral nutrition, oncology, specialty, and COVID medications.
- 196
- Monographs
- 37
- New stability monographs
- 808
- References and communication documents
- 2
- Editors with 13 contributing writers
Safely extend beyond-use dating of parenteral medications

Quickly access extended stability information for IV solutions
Safely extend dating of parenteral medications

Minimise medicines waste from partial dosing or temperature excursions

Reduce medicines costs

Allows medicines to be prepared in bulk or ahead of time

Enable optimal patient administration

Links to ASHP Injectable Drug Information

User-friendly data in easy to digest tables
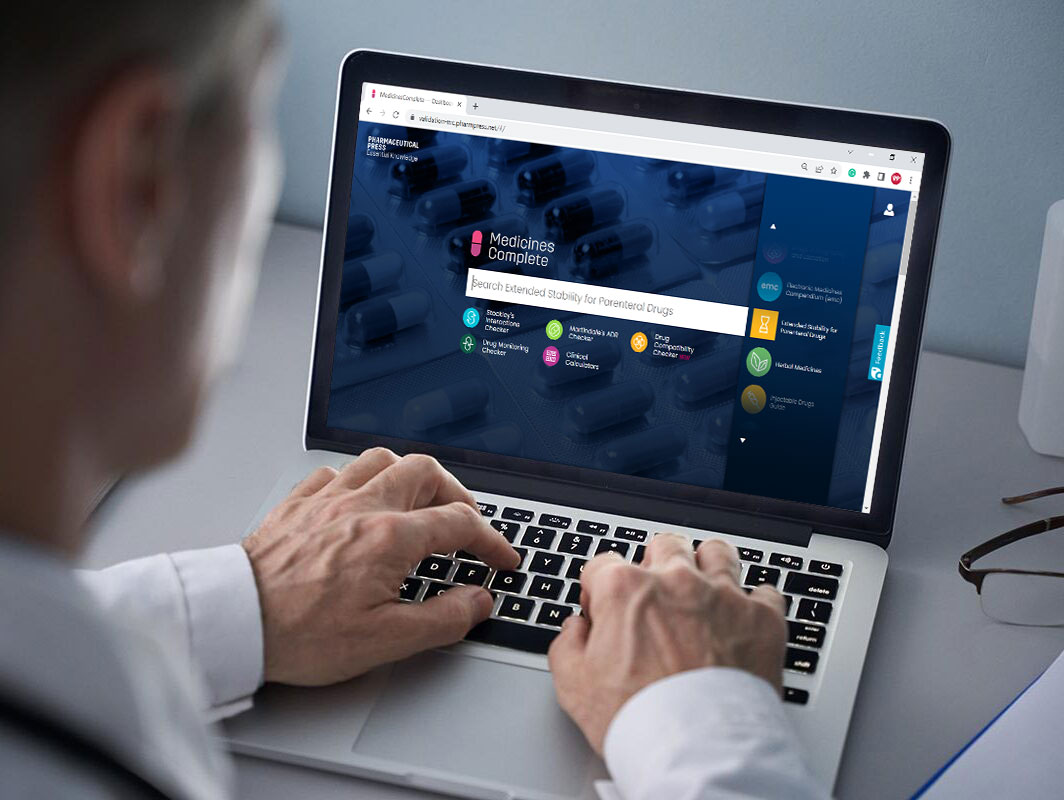
Request access today
Access Extended Stability for Parenteral Drugs through MedicinesComplete today.
Contact us now for pricing and access information.
Related publications
-
 BEST SELLER
BEST SELLERClinical Pharmacy Pocket Companion Second Edition
An A–Z handbook containing tools, suggestions and advice for administering pharmaceutical care.
Info -
 FEATURED
FEATUREDDale and Appelbe’s Pharmacy and Medicines Law Twelfth Edition
Guide to law and ethics for pharmacy practice in Great Britain
Info -
 BEST SELLER
BEST SELLERRules and Guidance for Pharmaceutical Distributions (The MHRA Green Guide) 2022
The reference for medicine distributors in Europe and the UK
Info
See all our printed publications in the Shop.





